VLADDIN Certificated Products
Great News! Vladdin has submitted 3 products to FDA to start the PMTA process, has been successfully received by FDA!
Vladdin has been preparing for PMTA since 2017, use the highest standard in the indurstry to build our factory, product design and quality control.
We have submitted our Vladdin Chopin, Vladdin Vantage, Vladdin X to PMTA review, our submission documents has been successfully received by FDA.
Here we express our sincere gratitude to all our supporters and partners, together we are here.
Overview of PMTA
A Premarket Tobacco Product Application (PMTA) can be submitted by any person for any new tobacco product seeking an FDA marketing order, under section 910(b) of the Federal Food, Drug, and Cosmetic (FD&C) Act. A PMTA must provide scientific data that demonstrates a product is appropriate for the protection of public health. In order to reach such a decision and to authorize marketing, FDA considers, among other things:
- Risks and benefits to the population as a whole, including people who would use the proposed new tobacco product as well as nonusers;
- Whether people who currently use any tobacco product would be more or less likely to stop using such products if the proposed new tobacco product were available;
- Whether people who currently do not use any tobacco products would be more or less likely to begin using tobacco products if the new product were available; and
- The methods, facilities, and controls used to manufacture, process, and pack the new tobacco product.
APMTA includes:
- Full reports of all information published or known to, or which should reasonably be known to, the applicant concerning investigations which have been made to show the health risks of such tobacco product and whether such tobacco product presents less risk than other tobacco products.
- Full statement of the components, ingredients, additives, and properties, and of the principle or principles of operation.
- Full description of the methods used in, and the facilities and controls used for, the manufacture, processing, and when relevant, packing and installation.
- An identifying reference to any tobacco product standard, if applicable.
The Tobacco Control Act
The Tobacco Control Act requires manufacturers of new or modified tobacco products to submit a premarket application and obtain a market authorization order before they market their products. To introduce a new or modified tobacco product, an applicant must submit an application to FDA providing information on the product sufficient to allow the agency to determine that an order authorizing the product’s introduction is appropriate for the protection of the public health.
To market a new tobacco product, Premarket Tobacco Application must be submitted under Section 910 of the Food, Drug, and Cosmetic Act. Before marketing a product in the United States, written notification must be received from FDA permitting the marketing of the new tobacco product. (Section 910 (b)).
PMTA Review Process
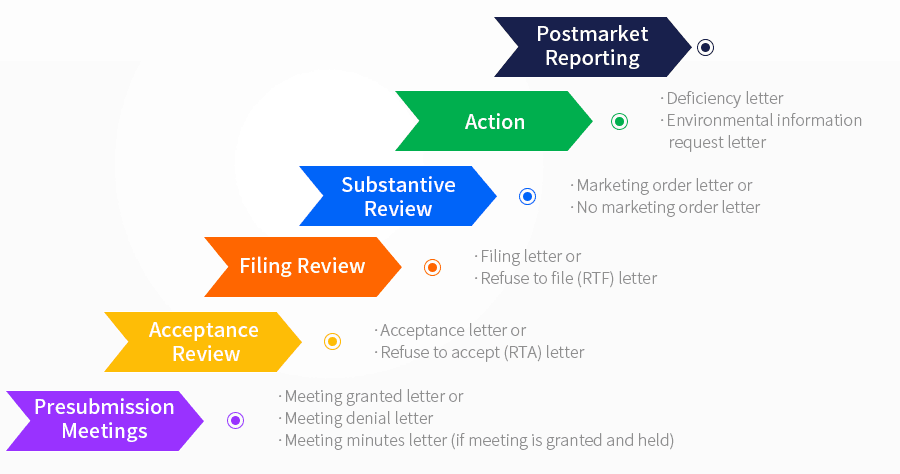
PMTA DEADLINE
The current deadline for PMTAs is May 12th, 2020. This date has changed multiple times since the initial publication of the deeming regulations, with the original date of August 8th 2018 being pushed back four years to 2022, before being recently brought forward after a district court decision. For companies which submit applications, the product can remain on the market for up to one year or until a decision is made.
The value of PMTA
Because FDA’s new comprehensive tobacco regulatory plan places nicotine at the heart of tobacco control efforts, the agency aims to authorize new tobacco products that will provide adults, who are addicted to nicotine, with less harmful alternatives to smoking cigarettes, while keeping any form of nicotine out of the hands of children. ENDS manufacturers are encouraged to create innovative, potentially less harmful tobacco products that can efficiently deliver satisfying levels of nicotine to addicted adult smokers, with less of the known toxicity than combustible products.