What is pmta vape?
A Premarket Tobacco Product Application (PMTA) can be submitted by any person for any new tobacco product seeking an FDA marketing order, under section 910(b) of the Federal Food, Drug, and Cosmetic (FD&C) Act. A PMTA must provide scientific data that demonstrates a product is appropriate for the protection of public health.
In short,A PMTA is a premarket tobacco application, or an application manufacturers have to make to the FDA to continue marketing and selling their products. 99% of all vaping products will have to go through this process. This includes importers of flavor and nicotine level an e-juice , vape device and other vape accessories.


What is pmta deadine?
The current deadline for PMTAs is May 12th, 2020. This date has changed multiple times since the initial publication of the deeming regulations, with the original date of August 8th 2018 being pushed back four years to 2022, For companies which submit applications, the product can remain on the market for up to one year or until a decision is made. The deadline for final decision was changed to Sept.9 2020.

What happens after pmta deadline?
Now that the deadline has passed, Vape product that had not been given premarket authorization by the FDA could no longer be offered for sale in the United States. And you won’t be able to purchase any products for which premarket authorization applications were not been submitted. Any product that did not go through the PMTA process is no longer available for purchase.
Preparing a PMTA
PMTA includes:
- Full reports of all information published or known to, or which should reasonably be known to, the applicant concerning investigations which have been made to show the health risks of such tobacco product and whether such tobacco product presents less risk than other tobacco products.
- Full statement of the components, ingredients, additives, and properties, and of the principle or principles of operation.
- Full description of the methods used in, and the facilities and controls used for, the manufacture, processing, and when relevant, packing and installation.
- An identifying reference to any tobacco product standard, if applicable.

How to Submit a PMTA
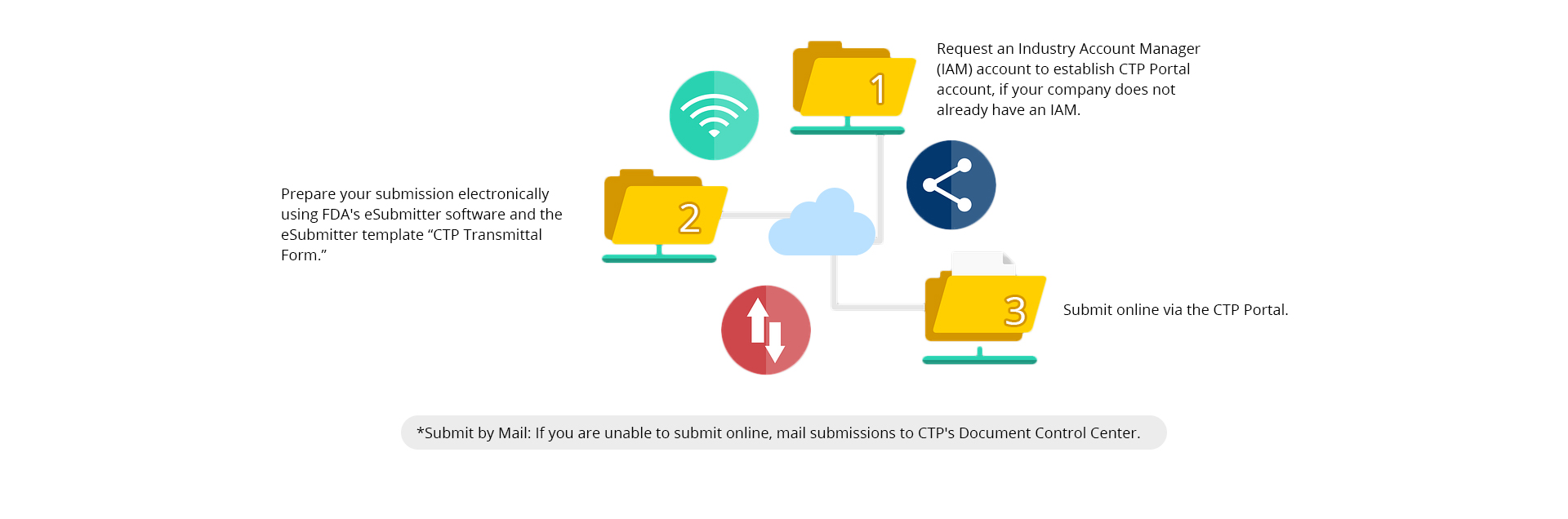
PMTA Review Process
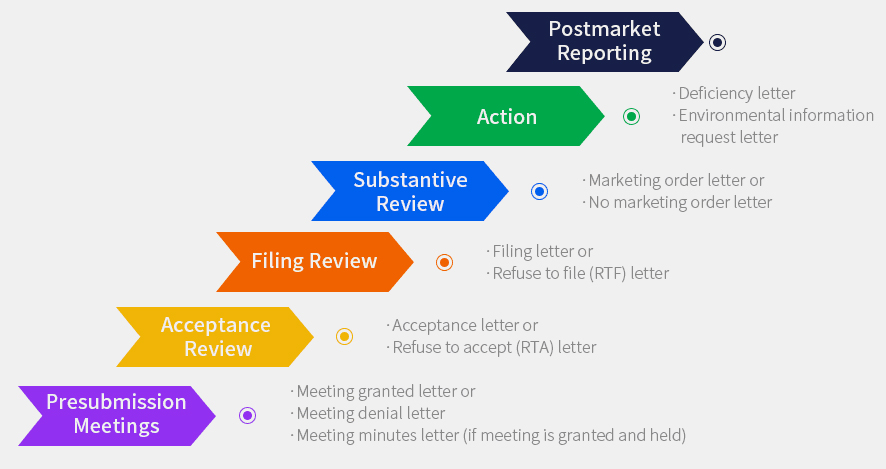

What does pmta mean for vaping?
For vapers in general, there are some substantial changes on the horizon. It’s estimated that each PMTA will cost $117,000 to $466,000. That is for every product you want to keep on the market, including different e-juice flavors and nicotine levels. For many businesses, this is an insurmountable hurdle. But going through the PMTA process that you will still be able to get quality vaping products.
Because FDA’s new comprehensive tobacco regulatory plan places nicotine at the heart of tobacco control efforts, the agency aims to authorize new tobacco products that will provide adults, who are addicted to nicotine, with less harmful alternatives to smoking cigarettes, while keeping any form of nicotine out of the hands of children. ENDS manufacturers are encouraged to create innovative, potentially less harmful tobacco products that can efficiently deliver satisfying levels of nicotine to addicted adult smokers, with less of the known toxicity than combustible products.
Vladdin to PMTA
Vladdin has been preparing for PMTA since 2017, use the highest standard in the indurstry to build our factory, product design and quality control. We have submitted our Vladdin Chopin, Vladdin Vantage, Vladdin X to PMTA review, our submission documents has been successfully received by FDA.